Targeting ER dynamics: a novel strategy for antitumor immunity
KNOXVILLE, TN, March 04, 2025 /24-7PressRelease/ — A groundbreaking study has revealed that reticulon-4 (RTN4), a protein involved in regulating the endoplasmic reticulum (ER) membrane curvature, plays a pivotal role in pyroptosis, a form of programmed cell death. The research demonstrates how manipulating RTN4 can trigger pyroptosis in cancer cells, enhancing antitumor immune responses. By targeting RTN4, the ER membrane undergoes significant remodeling, leading to the formation of the typical “bubble” structures associated with pyroptosis. This discovery not only advances our understanding of the molecular mechanisms behind pyroptosis but also introduces a promising new approach for anticancer immunotherapy.
Pyroptosis, a highly inflammatory type of programmed cell death, has garnered attention for its potential in cancer treatment. Characterized by cell swelling and the formation of “bubble” structures, pyroptosis is closely linked to the dynamics of the endoplasmic reticulum (ER). Despite growing interest, the precise mechanisms by which the ER triggers pyroptosis remain poorly understood, and the development of effective small molecules to induce this form of cell death has been limited. Given the importance of this process in cancer progression, understanding ER-associated pyroptosis has become a critical area of research.
In a study (DOI: 10.1093/procel/pwae049) published on September 10, 2024, in Protein & Cell, researchers from Peking University and Peking University People’s Hospital elucidated a novel mechanism connecting ER membrane remodeling to pyroptosis. Their findings reveal that by targeting reticulon-4 (RTN4), a key regulator of ER membrane curvature, it is possible to induce pyroptosis in cancer cells and promote antitumor immunity.
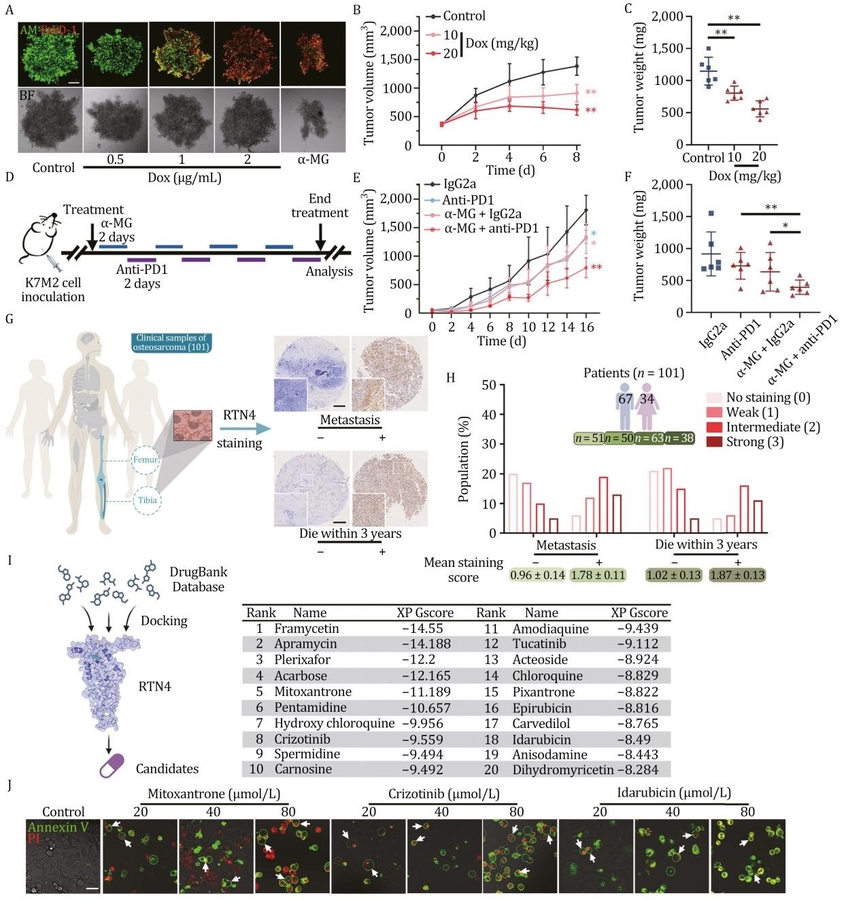
The researchers used a biotin-labeled chemical probe, α-mangostin (α-MG), to identify RTN4 as a crucial player in ER membrane curvature regulation. α-MG induces RTN4 degradation through the ubiquitin-proteasome system by recruiting the E3 ligase UBR5, triggering extensive ER remodeling. This degradation results in a shift in ER morphology from tubules to sheets, facilitating ER fusion with the plasma membrane and the formation of the typical “bubble” structures of pyroptotic cells. Further investigation revealed that RTN4 deficiency activates the caspase-3/GSDME pathway, driving pyroptosis. In vivo studies demonstrated that RTN4 knockdown significantly inhibited tumor growth and enhanced immune responses, particularly when combined with anti-PD-1 therapy. The study also highlighted α-MG as a potential small molecule degrader of RTN4, underscoring its therapeutic potential in cancer treatment.
Dr. Ke-Wu Zeng, one of the study’s corresponding authors, emphasized the transformative nature of these findings: Our research identifies RTN4 as a critical regulator of pyroptosis through its role in ER membrane dynamics. Targeting RTN4 can induce a potent antitumor immune response, offering a prospective strategy for anticancer immunotherapy.
The discovery of RTN4 as a druggable target for pyroptosis opens new possibilities for cancer treatment. Small molecules such as α-MG, which degrade RTN4, could be developed into novel anticancer agents. Furthermore, the synergistic effect of RTN4 degradation with immune checkpoint inhibitors like anti-PD-1 therapy highlights the potential for combination therapies to improve antitumor efficacy. This research lays the foundation for future clinical applications targeting ER dynamics to combat cancer.
References
DOI
10.1093/procel/pwae049
Original Source URL
https://doi.org/10.1093/procel/pwae049
Funding information
This work was financially supported by National Natural Science Foundation of China (82325050), National Key R&D Program of China (2022YFC3501601), Beijing Municipal Natural Science Foundation (7232273), Jinan New 20 Policies for Higher Education Funding (202228048), and Natural Science Foundation of Shandong Province (Joint Foundation for Innovation and Development) (ZR2022LZY021).
About Protein & Cell
Protein & Cell is a fully open access, peer-reviewed journal that publishes research concerning the latest developments in multidisciplinary areas in biology and biomedicine, with an emphasis on protein and cell research. Subject areas include biochemistry, biophysics, cell biology, developmental biology, genetics, immunology, microbiology, molecular biology, neuroscience, oncology, protein science, structural biology and translational medicine. In addition, Protein & Cell addresses research highlights, news and views, and commentaries covering research policies and funding trends in China, and provides a forum to foster academic exchange among researchers across different fields of the life sciences.
Chuanlink Innovations, where revolutionary ideas meet their true potential. Our name, rooted in the essence of transmission and connection, reflects our commitment to fostering innovation and facilitating the journey of ideas from inception to realization.
Related Link:
http://chuanlink-innovations.com
—
For the original version of this press release, please visit 24-7PressRelease.com here